
In a battery or other source of direct current the anode is the negative terminal, but in a passive load it is the positive terminal. When Thomson’s data are converted to SI units, the charge-to-mass ratio of the particles in the cathode-ray beam is about 10 8 coulomb per gram. What is the charge to mass ratio of cathode rays?īy definition, one coulomb is the charge carried by a current of one ampere that flows for one second: 1 C = 1 amp-s. He named his discovery “protons” based on the Greek word “protos” which means first. During this period, his research resulted in a nuclear reaction which led to the first ‘splitting’ of the atom, where he discovered protons. The proton was discovered by Ernest Rutherford in the early 1900’s. Which gas is used in cathode ray experiment?įor better results in a cathode tube experiment, an evacuated (low pressure) tube is filled with hydrogen gas that is the lightest gas (maybe the lightest element) on ionization, giving the maximum charge value to the mass ratio (e / m ratio = 1.76 x 10 ^ 11 coulombs per kg). The cathode is the source of electrons or an electron donor. The cathode attracts cations or positive charge. The cathode is the negatively charged electrode. Thomson is credited with the discovery of the electron on the basis of his experiments with cathode rays in 1897, various physicists, including William Crookes, Arthur Schuster, Philipp Lenard, and others, who had also conducted cathode ray experiments claimed that they deserved the credit.

Who discovered the electron?Īlthough J.J. The rest mass of the electron is 9.1093837015 × 10 − 31 kg, which is only 1/ 1,836the mass of a proton. It carries a negative charge of 1.602176634 × 10 − 19 coulomb, which is considered the basic unit of electric charge. Which is lightest particle?Įlectron, lightest stable subatomic particle known. Application of a sufficiently high electrical potential creates alkali or alkaline earth ions and their emission is most brightly visible at the anode. Are anode rays visible?Īn anode ray ion source typically is an anode coated with the halide salt of an alkali or alkaline earth metal. To release electrons into the tube, they must first be detached from the atoms of the cathode. … Cathode rays are so named because they are emitted by the negative electrode, or cathode, in a vacuum tube. Is cathode a ray?Ĭathode rays (also called an electron beam or an e-beam) are streams of electrons observed in vacuum tubes. Since the deflection of particle 3 is the maximum, it has the highest charge to mass ratio. The charge to mass ratio (emf) is directly proportional to the displacement or amount of deflection for a given velocity. Thomson proposed the plum pudding model of the atom, which had negatively-charged electrons embedded within a positively-charged “soup.” Which has highest charge-to-mass ratio? Thomson’s experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. In 1897, the mass-to-charge ratio of the electron was first measured by J. In the 19th century, the mass-to-charge ratios of some ions were measured by electrochemical methods. Thomson measured the mass of cathode rays, showing they were made of particles, but were around 1800 times lighter than the lightest atom, hydrogen.
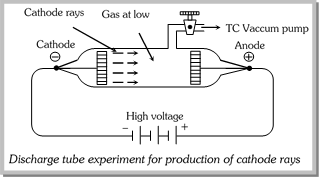

What is difference between cathode and canal rays?.What is the charge to mass ratio of cathode rays?.Which gas is used in cathode ray experiment?.Which has highest charge-to-mass ratio?.


 0 kommentar(er)
0 kommentar(er)
